Using Your Own Words Describe Grahams Law
He has twice been to the Millennium Stadium Cardiff as referee of a Football League playoff Final and at a EFL Trophy Final. Graham measured the rate of.

Graham S Law Diffusion And Effusion Definition Formula
Grahams law states that the rate of diffusion of a gas is inversely proportional to the square root of its molecular weight.
. In other words the facts and circumstances related to the use of force should drive the analysis rather than any. This page provides work in the use of Grahams Law of Effusion. Lindsey Grahams own words against him in a scathing new Lindsey Must Go ad Saturday.
When you press New Problem a question will be displayed to the right of the table. The rates of diffusion or effusion of gases under identical conditions is inversely proportional to square root of their vapour density. The flow of a gas through a small orifice at such a density that the mean distance between the molecules is large compared with the diameter of the orifice effusion.
Updated on July 03 2019. Rate 1 is the rate of effusion of the first gas. M m v v.
Grahams law in British English noun the principle that the rates of diffusion and effusion of a gas are inversely proportional to the square root of its density proposed by Thomas Graham in 1831. A scientific statement that is found to apply to a class of natural occurrences. Determine the relative rate of diffusion for krypton and bromine.
Grahams law also applies to effusion the process in which gas molecules flow through a small hole in a container. Its operation is very straightforward. Rate 1 is the rate of effusion for the first gas.
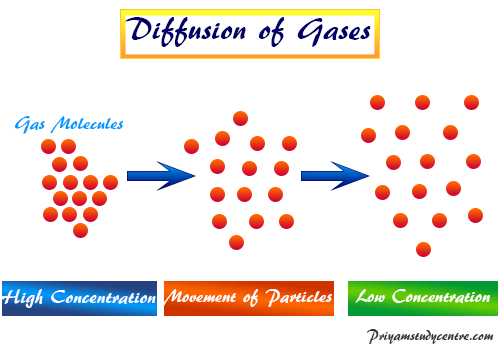
You can compare the rates at which two gases diffuse using Grahams law. Diffusion occurs spontaneously on its own. Mathematically This law is used in comparing the rates of diffusion or effusion of gases under similar conditions.
Where v velocity and m mass. This law gives better results at low pressure. At constant temperature the volume of a fixed amount of a gas is inversely proportional to its pressure.
Thus if the molecular weight of one gas is four times that of another it would diffuse through a. Based on his experiments with density he formulated Grahams law of effusion or diffusion which states that the rate of effusion or diffusion of gases at the same temperature and pressure are inversely. In1829 Thomas Graham a Scottish Chemist formulated the Grahams Law of the Diffusion and Effusion of Gases.
Look it up now. This law in practice is shown in Figure 53. A law stating that the rates of diffusion and effusion of a gas are inversely proportional to the square root of the density of the gas.
Rate 2 is the rate of effusion for the second gas. Graham Laws born 17 August 1961 in Whitley Bay Northumberland is an English association football referee who operates in the English Football League and has previously been a fourth official in the Premier League. Plots of Boyles Data.
Under constant pressure and temperature two gases diffuse into each other at rates inversely proportional to the square roots of their respective molecular weights or densities. Grahams research on the diffusion of gases was triggered by his reading about the observation of German chemist Johann Döbereiner that hydrogen gas diffused out of a small crack in a glass bottle faster than the surrounding air diffused in to replace it. The Kinetic Molecular Theory and Grahams Laws Thomas Graham The kinetic molecular theory can be used to explain the results Graham obtained when he studied the diffusion and effusion of gases.
This is from a 1993 appearance on ABCs Good Morning America. Grahams law of diffusion and effusion. A claim of excessive force by law enforcement during an arrest stop or other seizure of an individual is subject to the objective reasonableness standard of the Fourth Amendment rather than a substantive due process standard under the Fourteenth Amendment.
Grahams Law is a relation which states that the rate of the effusion of a gas is inversely proportional to the square root of its density or molecular mass. This law was proposed by Scottish physical chemist Thomas Graham in 1848. Diffusion is the process of slowly mixing two gases together.
That I was faithful to God and that I was faithful to the Scriptures which I believe have been inspired of God. Formula for Grahams Law of Diffusion and Effusion. Grahams law is a gas law which relates the rate of diffusion or effusion of a gas to its molar mass.
Effusion is the process in which the gas molecules are moved through the tiny hole in the container. Effusion is the process that occurs when a gas is permitted to escape its container through a small opening. It says that diffusion and effusion of gas molecules are in an indirect.
Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the molar mass of its particles. A B B A. M 1 is the molar mass of gas 1 M 2 is the molar mass of gas 2.
And I believe that they are our guide for Christian living and theyre our guide for society as well. Medical Definition of Grahams law. Rate1 Rate2 M2 M1 12.
It gives the relationship between effusion and diffusion of a gas to its molar mass. Billy GrahamI want to be. Heres a little bit of the making of Grahams law.
Diffusion is the movement of a substance from an area of higher concentration to an area of lower concentration. Grahams law of effusion also called Grahams law of diffusion was formulated by Scottish physical chemist Thomas Graham in 1848. A statement in chemistry.
Rate of diffusion of a gas is inversely related to the square root of its molar mass The equation shows the ratio of Gas As speed to Gas Bs speed. Learn about Grahams Law including the processes of diffusion and effusion and explore how to use the law to solve problems. Grahams Law helps explain how gas particles move through the air.
A body of rules regulations and legal opinions of conduct and action that are made by controlling authority and are legally binding. The question may require that you do a calculation and enter a numeric value or it may be multiple choice. According to this Law the rate of Diffusion of different gases at a constant temperature is inversely proportional to the square root of its density.
This relationship between pressure and volume is known as Boyles law after its discoverer and can be stated as follows. The conservative Lincoln Project launched Sen. It attacks his hypocrisy in his push to quickly confirm a new Supreme Court justice to replace the late Ruth Bader Ginsburg.
Graham s Law Physics. This formula can be written as. The key to this explanation is the last postulate of the kinetic theory which assumes that the temperature of a system is proportional to the average kinetic energy of its particles and.

Graham S Law Diffusion And Effusion Graham S Law Of Diffusion Graham S Law Of Effusion

Graham S Law Diffusion And Effusion Definition Formula


Comments
Post a Comment